The-Kelvin-Scale
The-Kelvin-Scale section explores William Thomson, Lord Kelvin's life and his development of now familiar scale itself while the section on
Colour Temperature addresses The-Kelvin-Scale as it relates to light.
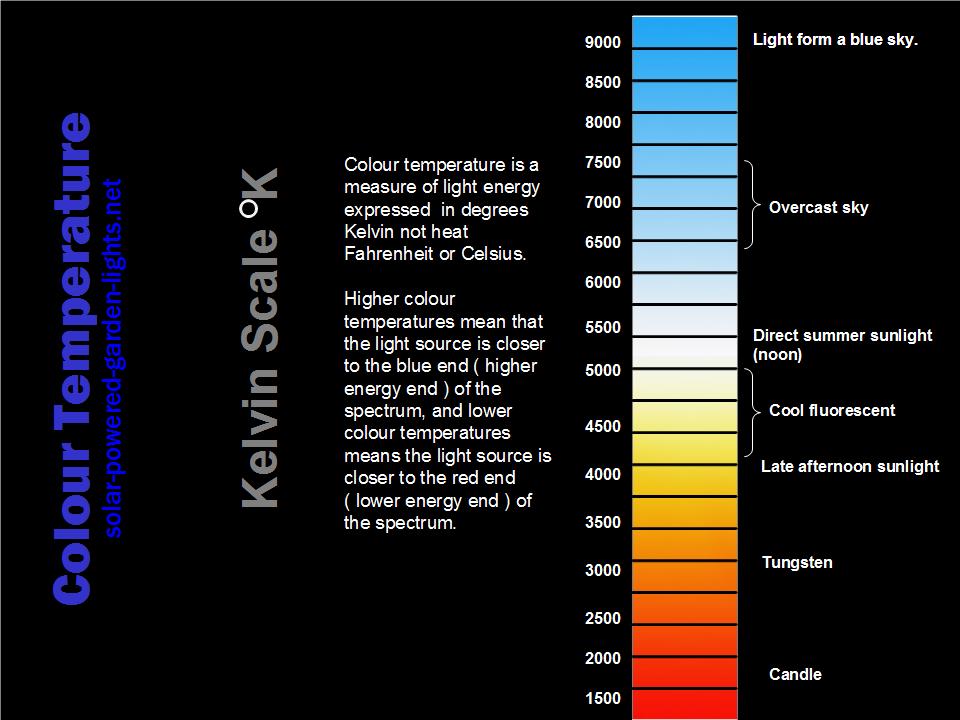 Kelvin-Scale/Colour Temperature
Kelvin-Scale/Colour Temperature
Thomson broadened the field of science and has to be regarded as a truly gifted human being.
Here is a condensed history to help become acquainted with the inventor of what we know today as the Kelvin Scale.
William Thomson, Lord Kelvin (1824-1907)
 Young William Thomson
Young William Thomson
William Thomson was born in Ireland in 1824. Following the death of their mother (born Margaret Gardner) both he and his brother James; in the company of their father (James Thomson), a mathematician; who had accepted a professorship at the University of Glasgow in Scotland.
Though only 8 and 10 years old respectively both boys attended their fathers lectures and at the ages of 10 and 12 William Thomson and his brother James began university studies. William Thomson was knighted in 1866 and in 1892, he was created the first baron Kelvin of Largs.
William Thomsons scale of absolute temperature takes its name from his appointed title and not directly from his family name. You may agree though; degrees Kelvin does seem to flow more easily off the tongue than degrees Thomson!
While Thomson was still in his teens he enrolled in post-graduate courses, first at Cambridge University, then at the University of Paris. In 1846 at the age of twenty-two, he was appointed to the position of professor of natural science at the University of Glasgow. He was also responsible for the construction of Britains first physics laboratory at the university.
It was Thomson`s work with James Prescott Joule Gases and their behaviour at low temperature that led to further interest in absolute temperature.
French physicist Jacques Charles found that for every degree centigrade (C) below zero that a gas is cooled, the volume of the gas would decrease 1/273 and suggests that at -273° C the volume of gas would be zero.
Being nothing more than a theory at the time, the general scientific community at the time could neither explain nor disprove the phenomenon. In 1848 Thomson explained the phenomenon: when the temperature of a gas is reduced, the energy level of its atoms is also reduced.
In the gas; as the atoms now move less they occupy less space which decreasing the volume of the gas. At -273° C the energy level of atoms reaches zero: the atoms stop moving. At this temperature the atoms occupy, almost no space, and their temperature cannot be lowered any further. Believing that this could be true for any substance theoretically, Thomson determined -273° C to be the absolute lowest temperature. Absolute zero.
Inventing the Absolute Scale
In 1848 Thomson applied his theory by inventing an absolute scale of temperature. As youll read again later on, the absolute scale is similar to the centigrade (Celsius) scale but extends the range of the scale by 273 units to include an absolute zero temperature. Zero and absolute zero coincide.The-Kelvin-Scale uses level absolute zero as its standard reference , rather than the freezing and boiling points of water which are the standard references from both the metric and imperial scales.
In the Imperial or English system the intervals are divided into 180 units or degrees Fahrenheit (°F).
In the metric system, the difference between freezing and boiling is divided into one hundred units or degrees Celsius (°C).
This one hundred degree range between boiling and freezing is identical to The-Kelvin-Scale but the two temperature scales differ by 273.15°.
The freezing point of water on the centigrade scale is 0° and the boiling point is 100°. The freezing point of water on the Kelvin Scale is 273° and the boiling point is 373°.
The freezing point of water on the Fahrenheit scale is 32° F and the boiling point is 212° F. Absolute zero is -460° F.
Thomson called his scale the absolute scale, but after his death it was renamed the Kelvin scale. If you enjoyed The-Kelvin-Scale then visit the Colour-Temperature section. It may be of interest to you as well.

New! Comments
Have your say about what you just read! Leave me a comment in the box below.